Abstract
Myeloproliferative neoplasms (MPNs) include three classical subtypes: polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). Since prefibrotic primary myelofibrosis (pre-PMF) was recognized as a separate entity in the 2016 revised classification of MPN, it has been a subject of debate among experts due to its indefinite diagnosis. However, pre-PMF usually has a distinct outcome compared with either ET or overt PMF. We conducted a retrospective study of MPN patients from October 2014 to June 2020 in the Fourth Affiliated Hospital of Zhejiang University. Patients who were diagnosed with ET, pre-PMF or overt-MF according to the 2016 WHO Classification were included. We reviewed the clinical parameters, haematologic information, and genetic mutations of patients using next-generation sequencing (NGS).
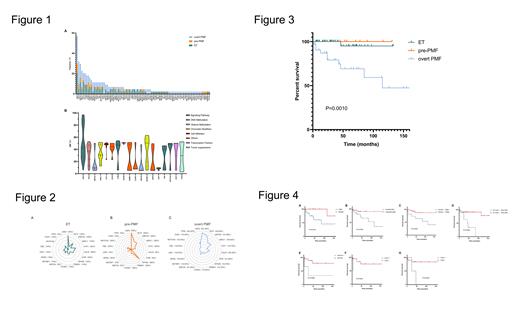
Mutation screening was performed in 44 patients by next‐generation sequencing techniques, 84 genes and 258 mutations were detected. JAK2 was the most frequently mutated gene (25/44, 56.82%), followed by TET2 (14/44, 31.82%), KMT2C (13/44, 29.55%), and ASXL1 (10/44, 23.73%) in MPN (Figure 1-A). The VAFs of all studied genes with mutation frequencies >10% are shown in Figure 1-B. Of the 20 patients with ET, 9 (45%) were positive for the JAK2 mutation, 5 (25%) carried FAT1, 5 (25%) carried KMT2C, and 4 (20%) carried CALR. Of the 5 patients with pre-PMF, 4 (80%) carried JAK2, 3 (60%) carried EP300, and 2 (40%) carried TET2. Of the 19 patients with overt PMF, 12 (63%) carried JAK2, 10 (53%) carried TET2, 7 (37%) carried ASXL1, and 6 (32%) carried KMT2C, as reported in Figure 2. The median follow-up was 36 months for ET, 42 months for pre-PMF, and 53 months for overt PMF. Overall survival between pre-PMF, overt PMF, and ET was significantly different (P<0.001), as shown in Figure 3. During the follow-up time, only one death of ET was registered, so we analysed the impact of clinical parameters and mutational status at diagnosis on outcome in PMF, including pre-PMF and overt PMF. We performed Kaplan-Meier curves to examine the relationships between the clinical parameters and patient survival. We found that male sex (P=0.0107), MPN10 symptoms (P=0.0354), anaemia (haemoglobin<120g/L, P=0.0239), and thrombocytopenia (platelet count <100 ×10 9/L, P=0.0002) were significantly related to inferior OS (Figure 4). Pre-PMF patients exhibited higher leukocyte counts, higher LDH values, a higher frequency of splenomegaly, and a higher incidence of hypertension than ET patients. On the other hand, pre-PMF patients had higher platelet counts and haemoglobin levels than overt PMF patients. Molecular analysis revealed that the frequency of EP300 mutations was significantly increased in pre-PMF patients compared with ET and overt PMF patients. In terms of outcome, male sex, along with symptoms including MPN10, anaemia, thrombocytopenia, and KMT2A and CUX1 mutations, indicated a poor prognosis for PMF patients.
In conclusion, we identified differences in the clinical, haematologic, and molecular presentations of ET, pre-PMF, and overt PMF patients, indicating that comprehensive evaluation of not only BM features but also clinical, haematologic, and molecular profiles is needed for accurate diagnosis and treatment of these three disease entities. The molecular analysis revealed that pre-PMF might be relevant to EP300 mutation, demonstrating the value of molecular examination. The results of this study indicated that comprehensive evaluation of BM features, clinical phenotypes, haematologic parameters, and molecular profiles is needed for the accurate diagnosis and treatment of ET, pre-PMF, and overt PMF patients.
Acknowledgment:The research was supported by the Public Technology Application Research Program of Zhejiang, China (LGF21H080003), the Key Project of Jinhua Science and Technology Plan, China (2020XG-29 and 2020-3-011), the Academician Workstation of the Fourth Affiliated Hospital of the Zhejiang University School of Medicine (2019-2024), the Key Medical Discipline of Yiwu, China (Hematology, 2018-2020) and the Key Medical Discipline of Jinhua, China (Hematology, 2019-2021).
Correspondence to: Dr Jian Huang, Department of Hematology, The Fourth Affiliated Hospital of Zhejiang University School of Medicine. N1 Shangcheng Road. Yiwu, Zhejiang, Peoples R China.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal